By Carly Anderson, Ph.D., Prime Movers Lab
I love thinking about quantum systems because they capture the complexity, uncertainty, and connectedness of the real world. In “classical” computing systems like a laptop or phone, every “bit” is either a 0 or 1 — there’s no in-between. However, a quantum bit, or “qubit,” can be part 0 and part 1 at the same time. Like a certaMeredith Brooks song, qubits can be “a little bit of everything, all rolled into one.”
As Chemical Engineer and & Partner at Prime Movers Lab, a deeptech venture firm investing in breakthrough science companies, I get to live on the front lines of deeptech innovation in quantum computing, climate tech, and other exciting areas. One of our core tenets is to share learnings from the amazing people and technologies we meet with the broader community. In this short piece, I’ll share examples of what quantum computing can enable in the context of chemistry and why it will be a powerful tool. I’ll also point out some of its limitations, and areas where technology is rapidly advancing with classical systems.
To frame this discussion, here is my high-level take on quantum computing for chemistry and materials applications based on what we’re seeing today:
- Quantum computers that can help us understand molecules larger than a handful of atoms are still 10-20 years away.
- Quantum computers will be faster at solving some chemistry problems but not all problems!
- The first quantum computers to be useful in modeling chemicals and materials will be hybrid systems with BOTH classical and quantum computer elements, and they will be run from the cloud.
How will quantum computing add value to the chemicals sector?
With the question of when we expect quantum computers to add value to the field of chemistry out of the way, let’s get into the how. Quantum computers are much better suited to simulate molecules and materials (and to find better ones!) than today’s “classical” computers. Why? Remembering back to Chem 101, molecules are atoms that are held together by shared (or stolen) electrons, which we call chemical bonds. The status of a particular electron in a molecule, and especially during a chemical reaction, has many possible values, each with some probability of being true — i.e., “a little bit of everything.”
On a classical computer, the equations to describe the location and movement of electrons take a HUGE amount of processing power and memory. Even for today’s supercomputers, using quantum mechanical equations to exactly calculate the properties of even simple molecules quickly becomes too complicated. Because of the limitations of classical computers, we can only get “exact solutions” for individual atoms and very small molecules like nitrogen (N2), water (H2O) and methane (CH4), which have just a handful of electrons in a few energy levels.
Quantum computers can encode much more complex information in a single quantum bit, or qubit, due to superposition. This allows them to solve the complex equations for electrons (and other quantum systems) more easily. It’s the difference between measuring derivatives over and over to find a minimum point in a mountain range and being able to see the entire landscape at once.
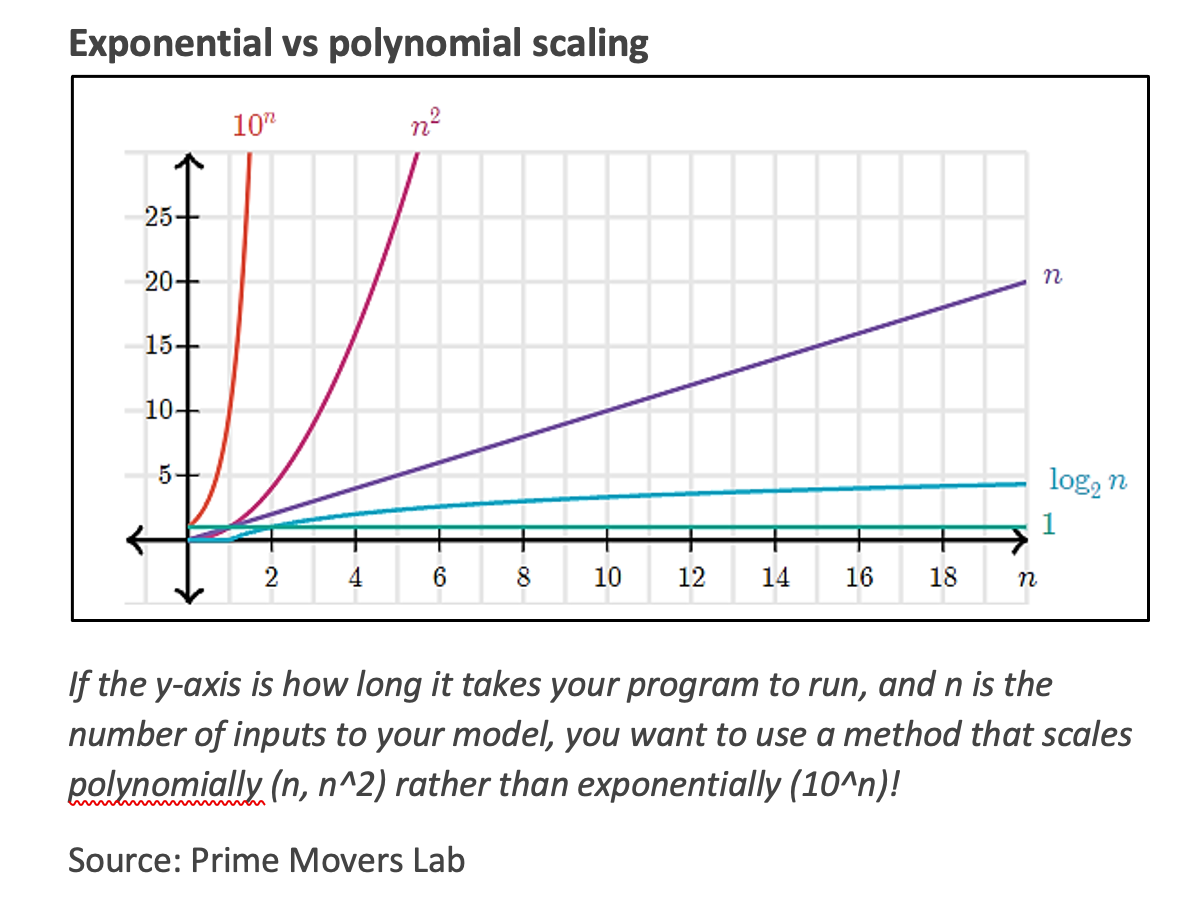
While quantum computers will speed up many chemical and materials calculations, the process still won’t be effortless. As the molecule being simulated gets bigger and more complex, more qubits and computational time will be required to determine accurate properties. The advantage for quantum computers is that the computation time generally scales only polynomially with the size of the problem vs. exponentially for classical computers, making large systems tractable. Polynomial time is much, much shorter than exponential time as n, the number of inputs or parameters to calculate (e.g., the number of electrons to include), increases. This relationship is illustrated below.
Quantum chemistry: already mature
It’s also important to remember that exact solutions (i.e., quantum computers) aren’t always needed to design new and better molecules. Quantum chemistry is a mature field. Researchers routinely calculate important chemical properties — ground-state energies, molecular geometries or what a spectrum will look like — by using approximations. Many of the approximations do a pretty decent job; scientists can comfortably calculate the chemical properties of molecules with a few hundred atoms within hours or days on a supercomputer.
The pace of computer-assisted drug discovery has in fact been accelerating for decades. The advent of density functional theory (DFT) for modeling quantum systems in the 1990s allowed chemists to make much more accurate structure predictions. Looking forward, the continued increase in the computation power, and the dawn of distributed cloud-based computational resources, will give chemists and biochemists more powerful tools. Dozens of startups, armed with machine learning and artificial intelligence routines, are on the hunt for better candidates for pharmaceuticals, battery materials, and even low-carbon cement formulations. Many such algorithms are producing good hits without using quantum computers.
The most exciting problems for quantum computers to work on are the ones where the approximations for classical computers break down. Prime examples of these are molecules that contain metal atoms (which have many associated electrons) and materials with large shared-electron systems. Examples of important chemical and biological molecules that are difficult to simulate with classical systems include:
- A DNA regulation enzyme histone demethylase, which helps switch genes on/off.
- The non-covalent binding between biotin and the avidin protein, which has been used for nanoscale drug delivery systems.
- Biological nitrogen fixation by the enzyme nitrogenase, which converts nitrogen into two ammonia molecules under ambient conditions.
- The N-chromophore photosynthetic complex, which plants use to efficiently convert sunlight and CO2 into organic molecules.
- Materials showing high-temperature superconductivity, which would be useful for applications in energy and electronics.
In addition to predicting the behavior of molecules with many electrons, quantum computers will also increase performance in modeling parts of molecules where super-high accuracy is key. This is particularly important in drug discovery studies (e.g., calculating the binding affinity of drug active regions in different liquids and protein environments).
Putting quantum computing in perspective
To zoom out and put computer-based chemistry in perspective: The ability to use quantum computers to create new, improved molecules is only the first step in the process. Let’s take the example of creating a vaccine for a new virus. Scientists use a range of computational tools to create a new molecule to bind to the receptor proteins on the virus’s surface, blocking it or tagging it for destruction. (Simulations like this are already used today, just with more approximate fits between the simulated molecules.) As we experienced in 2020, finding a great “in silico” molecule to block the receptor is just the beginning. Scientists then need to find a way to make it correctly and to keep it from falling apart. The company then needs to scale up production, ensure quality control, make sales, and secure distribution channels — likely many years of additional work. In the case of COVID-19, the tens of billions of public funds contributed, together with “warp-speed” production scale-up efforts, shortened this process to 15 months, a monumental feat.
This question of the value added by quantum computers vs the rest of the chemical discovery, scale-up, and commercialization process has big implications for the business models that quantum computing (and quantum computing software) companies can implement. Business models of selling hardware (less revenue), selling compute time on quantum computers (better), or quantum-computing-as-a-service are all possible but not equally good outcomes for quantum computing companies. Many instead hope to secure “profit-sharing” contracts similar to the relationship between 23andMe and GlaxoSmithKline, through which 23andMe receives 50% of the profits from any GlaxoSmithKline drug for a target identified using data from their health app. The value of the quantum computer and quantum software tools in creating the final product will certainly be the topic of heated discussions.
Tying quantum chemistry back to Sustainable Development Goals (SDGs), discovering new chemicals and routes to making others more sustainably will certainly be a boon to meeting multiple SDGs. For example, creating synthetic enzymes to make fertilizer could improve agricultural yields and crop nutrition (SDG 2, Zero Hunger). Faster, more precise drug discovery could help combat disease (SDG 3, Good Health and Well-Being).
My prediction is that we will experience the impact of quantum computers on drug discovery and medicine as a gradual improvement in patient outcomes, rather than a sharp step change. Cancer death rates have fallen by 31% in the past 30 years… in the next 30 years, a growing number of cancers will become a minor inconvenience. Quantum computers will further increase the number and the rate that new chemicals are discovered through “in silico” methods that have fantastic energy, industrial or agricultural applications. A word of caution is that these technologies will still face the same challenges and valleys of death that innovative companies see today. In a decade or two, quantum computers will give us the tools to answer previously unsolvable chemistry problems and will certainly lead to breakthrough discoveries. To fully take advantage of quantum computers, we need to apply them to the right problems, and also think critically about where the bottlenecks for breakthrough technologies are.
This article is one section of the report, “Quantum Impact — The Potential for Quantum Computing to Transform Everything.” Click here to learn more and access the full report.
Please see the PDF version of the full report for important disclosures.